7.5 Radioactivity
(Discovering the Universe, 5th ed., §4-5, §17-4)
 Nuclei with an excess of protons over neutrons, or that have many protons, tend to be unstable, since large number of protons can produce a long-ranged electric repulsion which exceeds the attraction of the short-ranged strong nuclear force. Nuclei with an excess of protons over neutrons, or that have many protons, tend to be unstable, since large number of protons can produce a long-ranged electric repulsion which exceeds the attraction of the short-ranged strong nuclear force.
Such nuclei may therefore split into two smaller nuclei with kinetic energy, a process called spontaneous nuclear fission.
Question: where does the kinetic energy come from?
Spontaneous nuclear fission is a form of radioactive decay, and such unstable nuclei are said to be radioactive.
Uranium (238U), for example, naturally decays into thorium (234Th) and helium (4He).
Question: what other radioactive materials have you heard of?
Extra: the Saturn-bound Cassini spacecraft is so distant from the Sun that it must rely on radioactive decay to generate power.
 Neutron decay is another form of radioactivity in which a neutron spontaneously decays into a proton, an electron, and a neutrino: Neutron decay is another form of radioactivity in which a neutron spontaneously decays into a proton, an electron, and a neutrino:
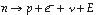
Neutron decay is a result of the fourth fundamental force in nature, the weak nuclear force.
Neutron decay can occur both in isolated neutrons and also inside nuclei (generally those that have many more neutrons than protons).
Question: after neutron decay in a nucleus, what happens to a nucleus' atomic number? its atomic mass?
- The rate at which a radioactive material decays is described by its half-life, the time it takes for half of the material to decay into its end products.
The half-life of a radioactive material is independent of the beginning amount.
Half-life can be used to judge the age of, for example, a meteorite, by comparing the relative amounts of a radioactive material and its decay products.
For example, the half-life of uranium (238U) is 4.5 Gy; rocks containing uranium commonly have an equal amount of its decay products, making them about 4.5 Gy old (assuming other sources of the decay products can be ruled out).
Question: what does that suggest about the age of our solar system?
- Many radioactive nuclei are isotopes of stable elements, with either an excess of protons or neutrons, and they commonly have half-lives of hours, days, or years.
The neutron has a half-life of only 17 minutes.
Because the Earth is very old, most of these materials have decayed away, and aren't generally found in nature.
- For any element with an atomic number greater than 83 (bismuth), all isotopes are radioactive.
Uranium, with an atomic number of 92, is the heaviest nucleus that exists in our solar system in large quantities, due to its long half-life.
Most of the other high-mass elements, however, have extremely short half-lives.
|