8.5 Sound
(Discovering the Universe, 5th ed., not!)
 Solids, liquids, and gases can all carry directed, energetic
disturbances through them, which are called sound. Solids, liquids, and gases can all carry directed, energetic
disturbances through them, which are called sound.
For example, when you speak, your vocal cords push against air
molecules, which bump into other air molecules, etc.
The sound moves through the air until it reaches a listener's
ear and pushes against their ear drum, which is then interpreted
by the brain.
- Sound is a type of wave, a natural phenomenon in which
something oscillates back and forth, passing energy along as
it does so.
For sound waves, it is the molecules which move back and forth
as they bump into their neighbors; the molecules themselves don't
travel very far.
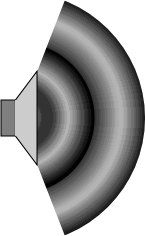
Because the molecules alternately get closer together and farther apart, the density oscillates as well.
In the picture at the right, the higher density regions are darker and the lower density regions are lighter.
For this reason sound is also called a density wave.
- Sound waves travel at a particular speed, the speed of
sound, that depends on the material, its density, and the temperature.
In air, it is about 340 m/s; it is generally faster in a liquid
and even faster in a solid, since the molecules don't have to
travel as far to bump into each other.
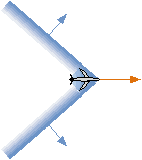
- If an object travels through a fluid faster than its speed
of sound, it pushes the fluid faster than it prefers to move.
As a result, the fluid is compressed into a narrow, dense layer
called a shock wave.
When produced by high-speed jet planes in air, this is also known
as a sonic boom.
An example of a (non-sound) shock wave are the bow waves produced
by a boat in water as it travels faster than the speed of water waves.
A shock wave travels at the speed of sound, and at an angle to
the motion of the causal object.
Picture Information

In the picture at the right, the "runaway" star
HD 77581 can be seen plowing through the interstellar medium
at 80 Km/s, producing a highly visible bow shock.
|